Zinc Reacts With Hydrochloric Acid
2KMnO 4 16 HCl 2KCl 2MnCl 2 5Cl 2 8H 2 O. It is practically insoluble in water and does not have any effect until it reacts with the hydrochloric acid in the stomach.
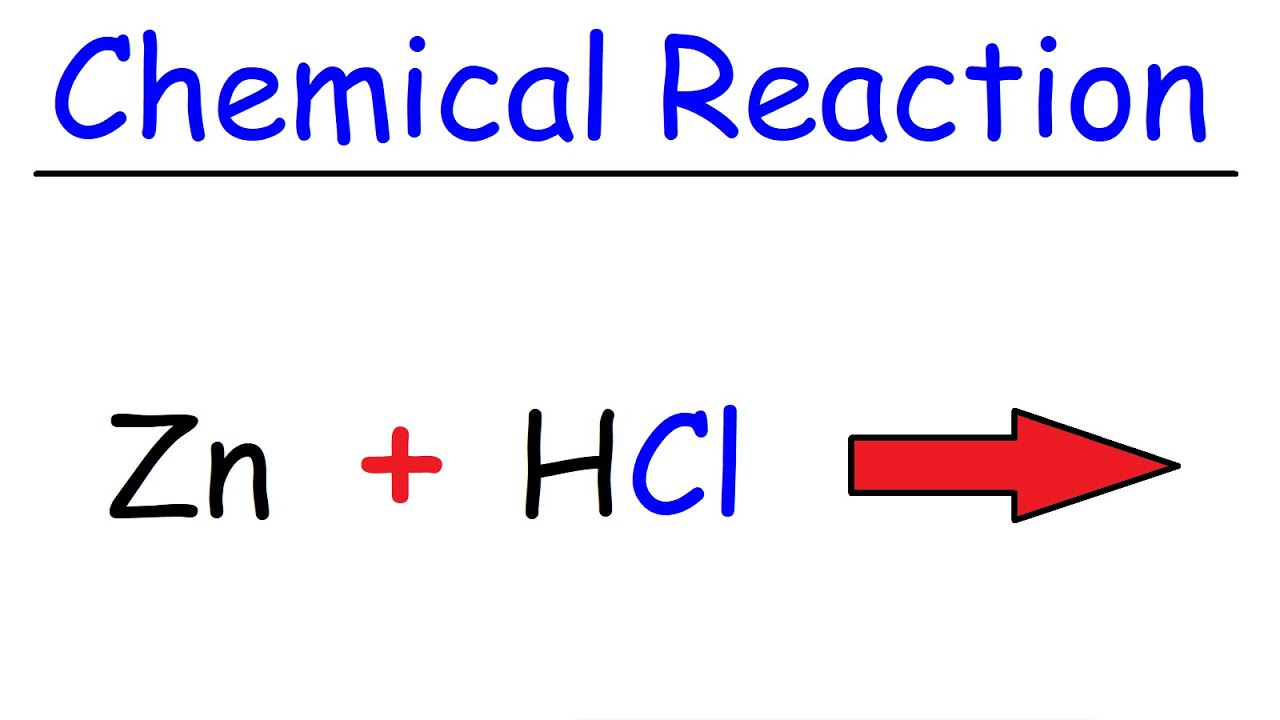
Zn Hcl Reaction Zinc Hydrochloric Acid Youtube
Reaction of Base with Metals.

. One example of this is the reaction between magnesium and hydrochloric acid. The reaction between an acid and a base to give a salt and water is known as a neutralisation reaction. Acid in water solution form is able to conduct electrical current.
Flask Erlenmeyer 250 mL each 50546 Hot plate 4-inch round 120 V each 1206701 Hydrochloric Acid Solution 60 N 11 500 mL 88449. Sodium aluminate and hydrogen gas are formed when sodium hydroxide reacts with aluminium metal. It is able to evolve hydrogen gas in the reaction with active metal like aluminum alkali metal alkaline metal zinc and so on.
Magnesium manganese and zinc liberate H 2. By the alchemist Jabir ibn Hayyan Geber by mixing common salt with vitriol sulfuric acid. Nitric acid reacts with most metals but the details depend on the concentration of the acid and the nature of the metal.
Lesson 1 is a series of test tube experiments in which each working group establishes as a common feature that hydrogen is given off as metals react with an acid if the metal reacts at all. Sulfuric Acid Safety Tips First Aid Safety Training Videos SDSs Hazards PPE. Chemical Properties of Hydrochloric acid HCl.
Hydrochloric acid is a strong corrosive acid thats formed by dissolving hydrogen chloride in water. Its called as electrolytes. Alkali Metal Salt Hydrogen.
It reacts with base to create the salt and water. And zinc plus sulphuric acid gives us zinc sulfate. Silver nitrate sodium chloride --- silver chloride and sodium nitrate.
When combined the resulting products are magnesium chloride and hydrogen gas. 2NaOH Zn Na 2 ZnO 2 H 2. If the gas bums with a pop sound then it confirms the.
Sodium hydroxide gives hydrogen gas and sodium zincate when reacts with zinc metal. Watch a cool science demonstration video to see the power of concentrated sulfuric acid on YouTube. You can also cause a double replacement chemical reaction when you combine an acid and a base.
They dont cancel. Test For Hydrogen Gas. Magnesium hydroxide enhances the integrity of the mucosal barrier of the stomach as well as improving the tone of both the gastric and.
As for bases they do not typically react with metals but there are a few metals that make exceptions such as zinc and aluminum. These reactions also result in salts and hydrogen gas. There it decreases the direct acid irritant effect and increases the pH in the stomach leading to inactivation of pepsin.
Watch sulfuric acid videos for safety training and chemistry experiments showing reactions. In general a neutralisation reaction can be written as Base Acid Salt W ater 215 Reaction of Metallic Oxides with Acids Activity 27 n Take a small amount of copper oxide in a beaker and add dilute hydrochloric acid slowly. It is recommended that you wear safety goggles and protective gloves during this step and that you rinse away any acid spills with cold tap water.
Zn 2 H Zn 2 H 2. Hydrogen gas and zinc chloride are formed when hydrochloric acid reacts with zinc metal. Reacts violently with acetic anhydride 2-aminoethanol ammonium hydroxide calcium phosphide chlorosulfonic acid 11-difluoroethylene ethylenediamine ethyleneimine oleum perchloric acid b-propiolactone propylene oxide silver.
Zinc Standard Solution 25 mgL as Zn 10-mL Voluette Ampule 16pkg 1424610 Zinc Standard Solution 1000 mgL 100 mL 1417742 Optional reagents and apparatus Description Unit Item no. Reactions that use an acid and a base as reactants is known as a neutralization reaction. Read a sulfuric acid safety guide here.
When alkali base reacts with metal it produces salt and hydrogen gas. Jabir discovered many important chemicals. History of Hydrochloric acid Hydrochloric acid HCl was first discovered around 800 CE.
Reacts with many metals including aluminum zinc calcium magnesium iron tin and all of the alkali metals to generate flammable hydrogen gas. Each working group needs a small selection of metals and acids to test. The gas evolved after reaction of acid with metal can be tested by bringing a lighted candle near it.
HCI has many uses and it is used in the production of chlorides fertilizers dyes textiles rubber and pharma. Also referred to as Hydrogen chloride and Muriatic acid. To prepare dilute hydrochloric acid solution slowly and carefully add approximately 100 ml concentrated hydrochloric acid 33 or 11 M to 900 ml of cold tap water.
HCl can be oxidized by potassium permanganate KMnO4 or potassium dichromate K2Cr2O7 liberated chlorine gas. Hydrogen gas and sodium sulphate are formed when sulphuric acid reacts with sodium metal. Mg 2 HNO 3 MgNO 3 2 Magnesium nitrate H 2 Mn 2 HNO 3 MnNO 3 2 ManganeseII nitrate H 2.
Dilute nitric acid behaves as a typical acid in its reaction with most metals. When this layer is corroded by acids such as hydrochloric acid and sulfuric acid the reaction proceeds with the evolution of hydrogen gas. 2K 2 Cr 2 O 7 14 HCl 2KCl 2CrCl 3 3Cl 2 7H 2 O.
Zinc reacts with alkalis as with acids. Hydrochloric Acid Synonyms for an aqueous solution of hydrogen chloride include chlorhydric acid hydrochloric acid and muriatic acid. Sulfuric acid barium hydroxide --- barium sulfate and water.
While the base possess some qualities as follow. With oxidants such as chalcogens and halogens Zn forms binary compounds such as ZnS and ZnCl 2. Sulfuric acid reacts violently with alcohol and water to release heat.
And the salt that we put on our food is produced when sodium reacts with hydrochloric acid. This should take around 40 minutes and most classes should be able to do this version. HCI is an inorganic liquid thats clear to slightly yellowish in color and reacts negatively with strong alkalis.
Since this is just a qualitative experiment it is not necessary to use. Hydrochloric acid reacts with salts like carbonates hydrogen carbonates sulphites etc. The Wurtzite structure showing the tetrahedral.
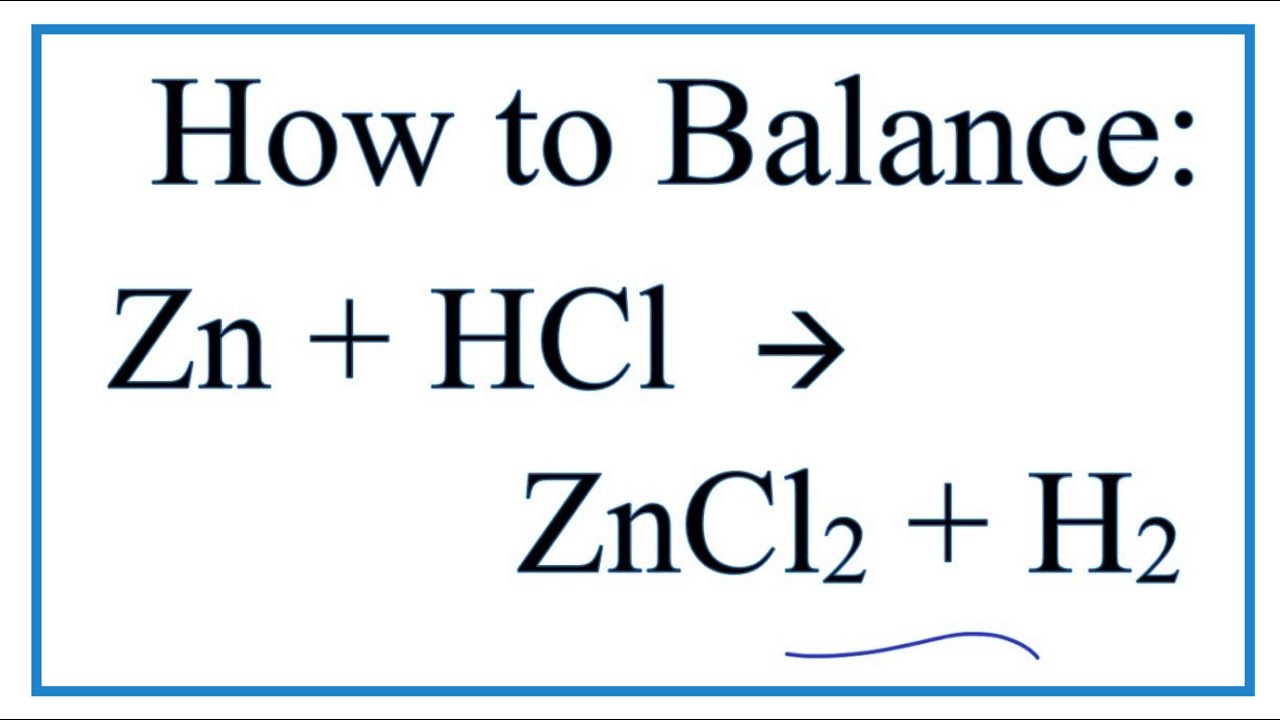
How To Balance Zn Hcl Zncl2 H2 Youtube
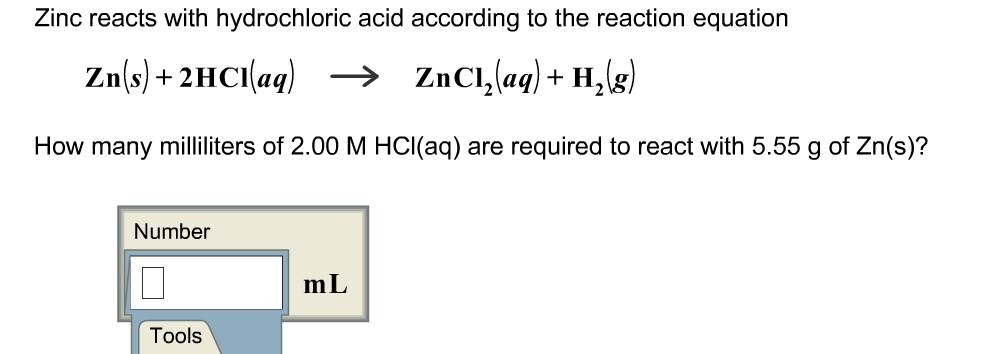
Solved Zinc Reacts With Hydrochloric Acid According To The Chegg Com

Assertion A When Zinc Is Added To Dilute Hydrochloric Acid Hydro

Zinc Reacting With Hydrochloric Acid Stock Image A500 0662 Science Photo Library
No comments for "Zinc Reacts With Hydrochloric Acid"
Post a Comment